Columbia-NYU-Takeda Alliance

Columbia University, New York University, and Takeda Pharmaceuticals have formed a collaborative research alliance to initiate and advance gastroenterology (GI) research programs with high potential to impact the development of new therapeutics for patients with GI disorders. Takeda is a global, value-based, R&D-driven biopharmaceutical leader headquartered in Japan, committed to bringing Better Health and a Brighter Future to patients by translating science into highly-innovative medicines. Takeda has its GI research laboratories in San Diego, California, and Cambridge Massachusetts. Our strategic collaboration is aimed at leveraging both the research & development capabilities and funding opportunities of Takeda to bridge the translational and commercial gaps of the Universities’ early-stage technologies. Takeda is seeking projects that are aligned to the GI Drug Discovery Unit’s discovery and translation strategies, and also forward-looking high-risk innovative concepts.
The objective of this collaboration is to advance sponsored research at the institutional level based on Request for Proposals mechanism. The award decisions will be made by a Joint Research Committee composed of Columbia, NYU, and Takeda representatives.
Questions? Please read FAQs below and/or reach out to the Columbia Alliance Manager, Mitchell Fullerton at [email protected].
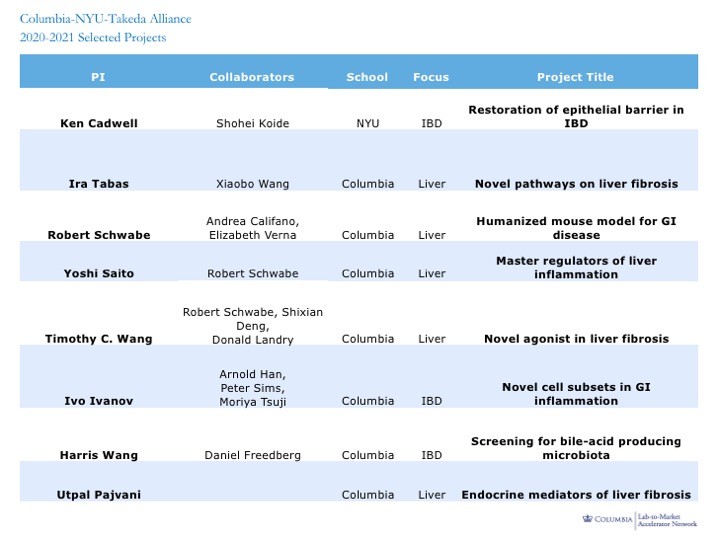
Selected Projects 2020-2021
FAQs
This collaborative research alliance has been established between Columbia University, New York University, and Takeda Pharmaceuticals. The purpose of this collaboration is to initiate and advance gastroenterology (GI) research programs with high potential to impact the development of new therapeutics for patients with GI disorders.
All faculty at NYU and Columbia with projects relevant to the GI Drug Discovery Unit’s strategic interests are open to apply.
$75,000 in direct costs for a ~1 year Pilot Project. Final budgets will be determined at the full proposal phase based on project needs.
Yes
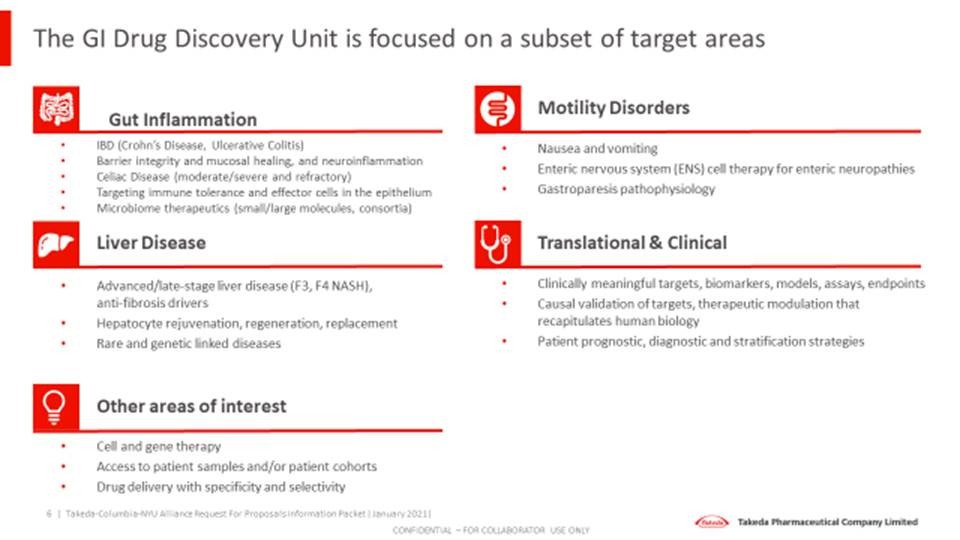
- Gut inflammation
- IBD (Crohn’s Disease, Ulcerative Colitis)
- Barrier integrity and mucosal healing, and neuroinflammation
- Celiac Disease (moderate/severe and refractory)
- Targeting immune tolerance and effector cells in the epithelium
- Microbiome therapeutics (small/large molecules, consortia)
- Liver disease
- Advanced/late stage liver disease (F3, F4 NASH), anti-fibrosis drivers
- Hepatocyte rejuvenation, regeneration, replacement
- Rare and genetic linked diseases
- Motility disorders
- Nausea and vomiting indications
- Enteric nervous system (ENS) cell therapy for enteric neuropathies
- Gastroparesis pathophysiology
- Translational & clinical
- Clinically meaningful targets, biomarkers, models, assays, endpoints
- Causal validation of targets, therapeutic modulation that recapitulates human biology
- Patient prognostic, diagnostic, and stratification strategies
- Other areas of interest
- Cell and gene therapy
- Access to patient samples and/or patient cohorts
- Drug delivery with specificity and selectivity
There are no specific requirements for project readiness.
Project selection occurs through two stages:
Stage 1: Non-confidential Letter of Intent (LOI) is submitted to a Joint Research Committee composed of Columbia, NYU, and Takeda representatives. These LOIs are reviewed to ensure there are no contractual, legal, or other encumbrances. The Committee will then review the LOIs and invite selectted applicants to develop a detailed Full Proposal.
Stage 2: For selected projects, Full Proposals will be developed and will contain detailed research protocols, a comprehensive budget, defined milestones, and equipment/other resources. The Joint Research Committee must approve the Full Proposal.
Please reach out to your Columbia Technology Ventures or NYU Technology Licensing Officer or Mitchell Fullerton ([email protected]) for additional information.
Investigators have the right to publish and publicly disclose results from the collaboration. However, the materials to be disclosed (e.g. manuscript, abstract, poster) must be provided to Takeda at least 30 days before disclosure to allow for a review of confidentiality and patentable IP.
Yes. Results from the collaboration can be used for non-profit and government entities.
About Takeda
Takeda is among the top 14 largest and 14th fastest growing pharmaceutical companies in the United States. They have more than 18,000 employees in the U.S. dedicated to helping patients across several parts of our business. Their U.S. Hub in Massachusetts is the operational center for the U.S. commercial business unit, Global R&D, Global Oncology, Global Vaccines, and biologics manufacturing.
